Ozone Overview
What is ozone?
ozone
| Etymology | Greek (OZEIN / smell) |
| molecular formula | O3 |
| molecular weight | 48 (Heavier than air, usually gas (boiling point -112°C)) |
| Unstable, reacts quickly, and then turns into oxygen. | |
| Strong oxidizing power (second only to fluorine in nature) |
Oxidizing power comparison table
| oxidizing agent | Oxidation-reduction potential (V) |
|---|---|
| Fluorine | 2.87 |
| ozone | 2.07 |
| chlorine | 1.78 |

Precautions when handling ozone
Tolerance
- 0.1 ppm Japan Society for Occupational Health (1985)
- 0.1ppm ACGIH (1961)
The arithmetic average of exposure levels when engaged in non-physically strenuous work during a workday of approximately 8 hours per day, 40 hours per week (some reversible changes are acceptable as long as they do not result in a decline in physical function).
However, caution is required even at concentrations below 0.1 ppm.
The effects of ozone
| Ozone (ppm) | action |
|---|---|
| 0.01~0.02 | You may notice a slight odor (but you will eventually get used to it). |
| 0.1 | It has a pronounced odor and can irritate the nose and throat. |
| 0.2~0.5 | Exposure for 3 to 6 hours will impair vision. |
| 0.5 | There is a clear irritation to the upper respiratory tract. |
| 1~2 | Two hours of exposure can cause headache, chest pain, dryness of the upper respiratory tract and coughing, and repeated exposure can lead to chronic poisoning. |
| 5~10 | Symptoms include increased pulse rate, body pain, and anesthesia, and continued exposure can lead to pulmonary edema. |
| 15~20 | Small animals die within two hours. |
| 50 | A human's life becomes endangered within an hour. |
Ozone is a powerful oxidizing agent consisting of three oxygen atoms, and its oxidizing power has the ability to disinfect and inactivate bacteria and viruses.
This has been well known for some time, but it has recently attracted particular attention due to the environmental impact of the residual by-products of conventional methods such as chlorine-based chemicals, formalin, and ethylene oxide.
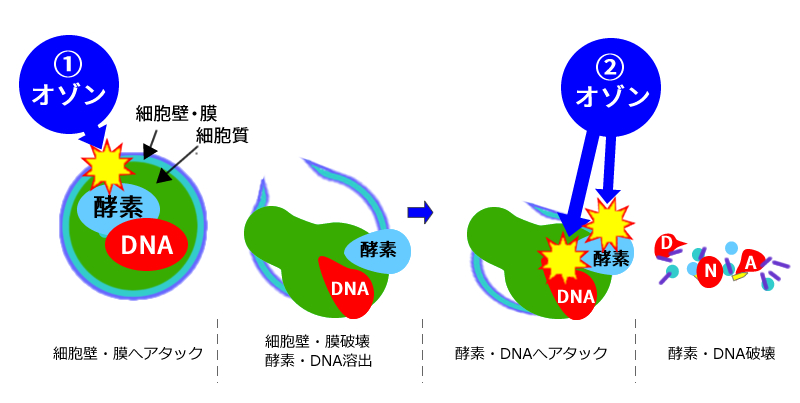
Ozone sterilization mechanism (bacteria)
| ① |
|
||||
| ② |
|
||||
| ① and ② are multi-point attacks ⇒ resistant bacteria cannot be developed | |||||
Characteristics of each sterilization method
| kinds | Features | Points to note | |
|---|---|---|---|
| gas | Ozone gas | Environmentally friendly material that returns to oxygen High disinfecting effect No by-products are produced |
Toxic, distinctive odor Residual gas treatment required (high concentration only) Metal corrosion and resin erosion (high concentrations only) |
| Ethylene oxide gas (EOG sterilization) |
Gas remains Often used in medical equipment No metal corrosion or resin erosion |
Toxic, carcinogenic, ether smell Residual gas treatment is essential |
|
| Formalin gas | Gas remains Use concentration is lower than ethylene oxide No metal corrosion or resin erosion |
Toxic, carcinogenic, pungent odor Residual gas treatment is essential |
|
| liquid | Ozone water | Environmentally friendly material that returns to oxygen High disinfecting effect No by-products are produced |
No residue Metal corrosion and resin erosion (high concentrations only) Volatile and ozone smell |
| hypochlorous acid | Used in various fields as a disinfectant High residual |
Generation of by-products such as organic chlorine compounds (trihalomethanes, etc.) | |
| Alcohol | High concentration and effective disinfection Volatile |
Volatile, rough hands Alcohol smell Flammable |
|
| others | UV rays | Experience in living environments and various fields No residue after lamp irradiation |
Be careful of the recovery of bacteria that have been eradicated |
Comparison of sterilization methods (gas)
| Ozone gas | Ethylene oxide gas | Formalin gas | |
|---|---|---|---|
| Sterilization mechanism | Eliminates bacteria and viruses by reacting with their DNA/RNA and proteins and oxidizing and decomposing them. | ||
| Usage concentration | low | high | Medium |
| Carcinogenicity | none | Yes | Yes |
| Residual properties on the target object | Almost none (self-decompose) |
Be careful of residual (Difficult to disassemble) |
Be careful of residual (Difficult to disassemble) |
| Ease of use | Easy | difficulty | difficulty |
Comparison of sterilization methods (liquid)
| Ozone water | Hypochlorous acid water | alcohol | |
|---|---|---|---|
| Sterilization mechanism | Structural destruction of cell walls and membranes Enzymes and DNA destruction No resistant bacteria |
- Enzyme damage (inhibition) Can cause resistant bacteria |
Enzyme damage (inhibition) Cell wall and membrane lipid destruction Can cause resistant bacteria |
| Usage concentration | 0.5 to several mg/L | 50mg/L or more | Approximately 50 to 90% |
| Impact on the environment, etc. | Almost none (self-decompose) |
Toxic chlorine gas By-product generation |
rough hands, food degeneration, |
| Ease of use | Easy | difficulty | Easy |
| Sterilization power | large | small | Medium |
Comparison of sterilization methods (other)
| Ozone gas | UV rays | |
|---|---|---|
| Sterilization mechanism | Destroys enzymes and DNA | Changes DNA structure |
| Carcinogenicity | none | Yes (skin cancer) |
| Antibacterial properties | No resistant bacteria | Be careful of the recovery of bacteria that have been eradicated |
| Disinfection range | Disinfects the inside of the object | Disinfects only the surface of the object |
| Impact on the environment, etc. | Almost none (self-decomposes) | none |
| Ease of use | Easy | Easy |
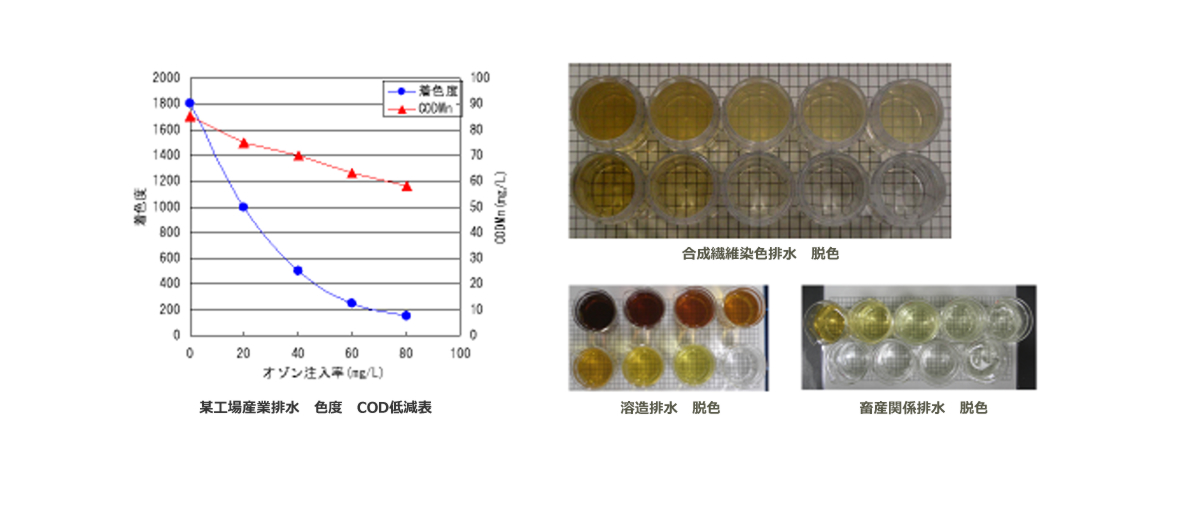
Ozone also has a decolorizing effect due to its strong oxidizing power.
bleaching effect
The decolorization effect is achieved by oxidatively decomposing the unsaturated double bonds in molecules that cause discoloration in domestic wastewater and industrial wastewater, changing the chemical structure.